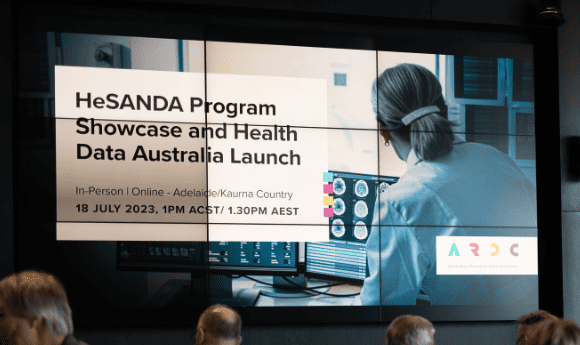
On 18 July 2023, Health Data Australia was launched to over 250 attendees online and at SAHMRI in Adelaide on Kaurna Country.
Health Data Australia is a new platform that makes clinical trial data easier to find and share. It’s the culmination of over 2 years of work by 72 health organisations from across Australia, coordinated by the ARDC.
Reusing Data to Accelerate Research and Better Health Outcomes
Clinical trials are a significant investment in terms of money. They also involve many hours of trial participants’ time. Therefore, we want to ensure that the data is used again for the maximum benefit to the health of Australians.
Through sharing data, Health Data Australia is accelerating the benefits to public health and the community.
Health Data Australia was created by the Health Studies Australia National Data Asset (HeSANDA) program. Coordinated by the ARDC, HeSANDA has 9 nodes which cover 72 health and medical research organisations, health service operators and clinical trial networks from across Australia. The HeSANDA partners co-designed the framework for sharing clinical trial data through Health Data Australia. In addition, more than 200 people also contributed to its development through working groups, incorporating feedback from research trial participants, consumers, researchers and trial organisers.

A National Partnership
The speakers at the launch shared their experience participating in HeSANDA, and their excitement to see Health Data Australia go live.

Professor Steve Wesselingh, Executive Director of SAHMRI said, “I think most Australians would expect that if we develop data utilising their money and often their time, if it happens to be a clinical trial, that data would then be available to others to ensure those assets are widely used to improve health of Australians. So HeSANDA, led by the ARDC, should really be congratulated on developing the infrastructure which enables discoverability, accessibility and re-use of health research data and such an important outcome.”

Delaine Smith, CEO of Australasian Leukaemia & Lymphoma Group (ALLG) and HeSANDA node lead for the National Cancer Cooperative Trials Groups node said, “The group has 100s of data sets that need to be shared. And now we can. Health Data Australia increases the value of clinical trials in Australia for Australia.”

Dr Kristan Kang, ARDC Program Manager for HeSANDA walked through how clinical trial data can be found and shared via the Health Data Australia platform. He emphasised that data remains with the owner of that data at all times and is not stored on Health Data Australia. Only the description of the data is held on the platform. The data request system allows the data owner to choose when and how to share data with another researcher. Dr Kang thanked the HeSANDA nodes, partner organisations, and all those who contributed to the consultation and design of the program.

Dr Adrian Burton, Deputy CEO and Director of People Research Data Commons, ARDC, said that the HeSANDA initiative is part of a multi-decadal initiative to support fundamental infrastructure for leading-edge research. The launch of Health Data Australia is only the beginning of the journey to unlock the potential for health studies data. The ARDC is growing its digital research services for the medical and health research community to tackle the key data challenges for health and medical research via the People Research Data Commons.

Wendy Keech, Executive Director of Health Translation SA and HeSANDA SA Node Lead said, “HeSANDA has been a great project. We still have a ways to go, but we’re on the journey and we’re really looking forward to what it can do for South Australia.”

Professor Julian Elliott from Cochrane Australia and the Australian Living Evidence Consortium said, “By making the outputs of research studies more widely available, we’re unlocking the potential to a much, much broader range of researchers. We can’t predict the limit to the use of those data. There is no limit to the imagination of others and the uses that they might put those data together. This is extremely exciting.”
Watch the recording of the HeSANDA program showcase and Health Data Australia launch:
Next Steps
With Health Data Australia now live, the HeSANDA network will continue to work with researchers to include more clinical trial datasets and continue to enhance the platform. We will also encourage researchers to search for data and request access to data on Health Data Australia. To find out how to upload your clinical trial data, contact us.
While HeSANDA is focusing on sharing clinical trial data, the ARDC is growing its digital research services for the medical and health research community. If you are involved in Health Cohort Research, please join our upcoming workshops.
Find clinical trial data on Health Data Australia.
The ARDC is funded through the National Collaborative Research Infrastructure Strategy (NCRIS) to support national digital research infrastructure for Australian researchers.
Here are some photos from the event. Images: ARDC / Andy Steven












Author
Reviewed by
Categories
Research Topic
Related Program
Related Projects
Related News & Events
Related Case Studies