
Reducing Deaths from Air Pollution
Exploreabout Reducing Deaths from Air Pollution
In partnership with the health research community, the ARDC is establishing national infrastructure to enable researchers to access and share data from health studies, with an initial focus on clinical trials.
The HeSANDA initiative aims to stimulate new data-driven research ideas, increase the impact of health research and ultimately improve the health and wellbeing of Australians.
A wealth of data is created through health research studies, including information about the people taking part in the research, their health and their response to interventions being studied.
The data collected in one study is extremely valuable to other studies. However, the challenges of patient privacy and the naturally siloed approaches of research groups and state jurisdictions have created a confusing array of inadequate options and cottage-industry approaches to data sharing. The ARDC is playing a critical role in partnership with the health research community to synchronise efforts, align approaches and build national data capability.
This untapped trove of health research data will bring immense value to health research, maximising the return on investment of past research and allowing future research to build upon it to improve health outcomes for Australians.
Similar capability has already been established in European health research infrastructure (ECRIN and EOSC Life) and data sharing platforms are emerging in the UK and USA. Australia risks being left behind without a corresponding capability.
The Advisory Committee for the initiative includes these key national health research organisations:
Informed by the NCRIS facilitation process, the HeSANDA initiative implemented an 18-month systematic consultation and co-design process with assistance from the Australian Institute of Health and Welfare (AIHW), clinical trialists, researchers, health consumers and research institutions, as well as infrastructure providers and policy makers involved in clinical trials research. This established consensus around the purpose, content and requirements for a national health data asset to be created through the HeSANDA initiative, summarised in the following figure:
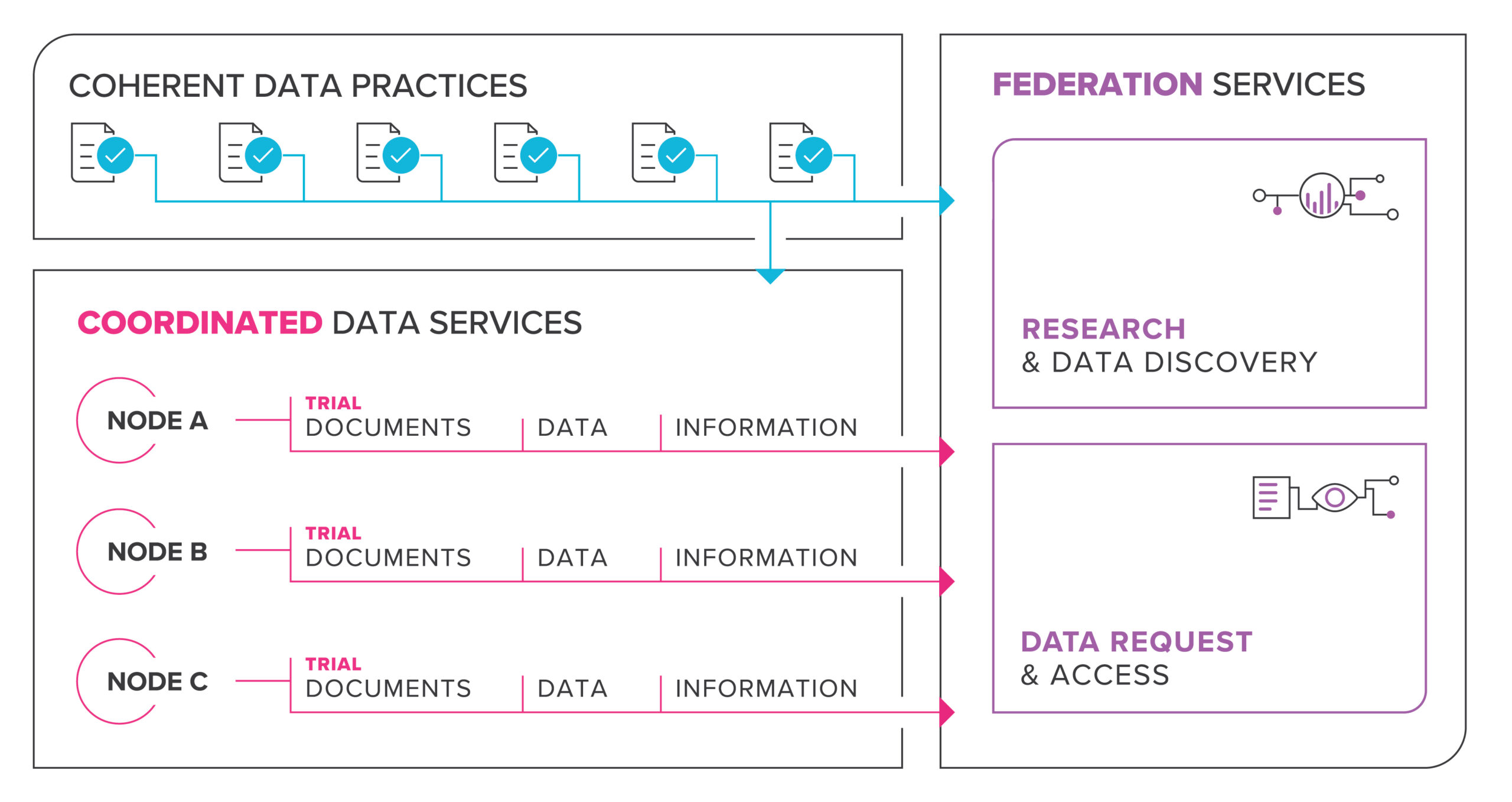
In June 2021, the HeSANDA initiative hit a significant milestone with confirmation of 9 initial nodes of a nationally distributed network of health data sharing infrastructure.
These 9 initial nodes confirm a broad national participation covering 72 health research organisations including:
The infrastructure build phase of this 9-node network commenced in 2021 with coordinated design, implementation and testing. The initial rollout of the data asset will contain clinical trials data generated from more than $56 million of public investment from the NHMRC and MRFF.

The broad alliance of partners in the HeSANDA initiative are committed to translational research.
Together they have a demonstrated track record of translating health research into positive changes to:
The new HeSANDA data sharing capability put in place by these node partners is designed to accelerate and transform such activities by providing a robust and coordinated national infrastructure.
“The Australian Health Research Alliance (AHRA) across our ten NHMRC accredited Centres, integrates healthcare, research and education to deliver better health. Our priorities include improving data standards, governance, access and use for research and healthcare improvement. We strongly support, and are partnering to deliver, the aims of HeSANDA, with nodes across Australia to enhance collaboration, knowledge and technology exchange, capability building and delivery of impact through better use of data,” said Prof Helena Teede, Chair of AHRA’s Data-Driven Healthcare Improvement Committee.
Phase 1 of the HeSANDA initiative has 9 nodes focused on clinical trials. Future phases of the initiative are designed to include more nodes and support high value data from other health study types, such as clinical registries or cohort studies.
“The ARDC has taken a highly collaborative approach to the HeSANDA initiative and we are glad to both represent and help capture the voice of the investigator-led clinical trials sector. ACTA supports the ethical, feasible and valid sharing of clinical trials data for use in further research, toward better health through best evidence,” said Prof Steve Webb, Chair of the Australian Clinical Trials Alliance (ACTA).